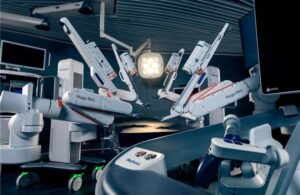
The landmark approval for the medtech giant is the first in the U.S. for its soft-tissue surgical robot. Medtronic unveiled Hugo in September 2019 as a potential competitor to long-time industry leader Intuitive Surgical. The company submitted the platform to the FDA for the urology indication in April. It has since met its endpoints in a hernia repair study and been put under evaluation in a study for gynecological procedures as well.
Outside the U.S., Hugo has been used in tens of thousands of urologic, gynecologic, and general surgery procedures in more than 30 countries across 5 continents. Now, Hugo’s approval brings a versatile robotic-assisted platform to U.S. surgeons and health systems seeking to expand soft-tissue robotic surgery programs and access to minimally invasive care, the company said in a news release.