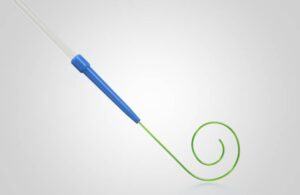
The medtech giant designed Stedi to enhance the performance of the transcatheter aortic valve replacement (TAVR) platform. It comes compatible with all commercially available TAVR systems for treating severe aortic stenosis. The guidewire has FDA clearance and is now available in the U.S.
Medtronic announced the launch of the new guidewire at the 2025 Transcatheter Cardiovascular Therapeutics (TCT) conference in San Francisco. It marks the latest development for the TAVR offerings at the company, following the recent announcement of a partnership with DASI aiming to optimize outcomes for patients undergoing TAVR.