The FDA Safer Technologies Program (STeP), a collaborative program, helps reduce the time needed to develop and earn marketing authorization for eligible devices. The FDA launched STeP in 2021, modeling the program on its breakthrough devices designation program.
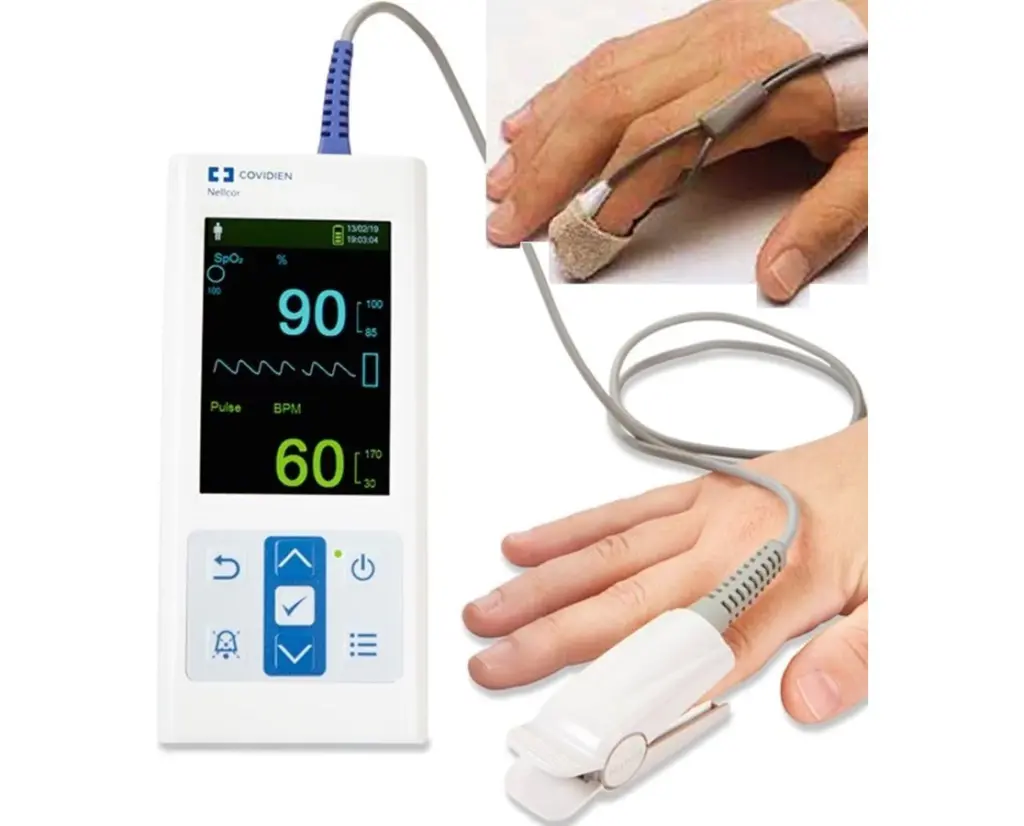
Now, Medtronic says its Nellcor pulse oximetry technology, currently in development, joins the program. The investigative Nellcor technology aims to integrate patient-specific and sensor-specific data into oxygen saturation calculations used in pulse oximetry. According to a news release, acceptance came based on Eligibility Criterion 2a. That outlines a reasonable expectation for the device to improve the benefit-risk profile and reduce the occurrence of serious adverse events.