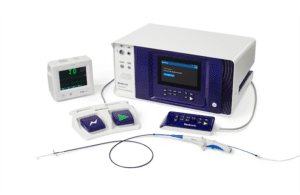
In addition, the medtech giant received European Union approval for its Nitron CryoConsole, which builds upon the legacy of the company’s cryo franchise with features to optimize the workflow for cryoballoon ablation.
The news of the regulatory wins in the EU comes about eight months after the medtech giant received a CE mark for its Affera mapping and ablation system, which offers both pulsed field and radiofrequency ablation.
Pulsed-field ablation is a non-thermal method for cardiac ablation that has the potential to positively disrupt the way atrial fibrillation (AFib) is treated. Companies including Medtronic, Boston Scientific, Johnson & Johnson’s Biosense Webster and more are competing to get PFA systems onto the market. Medtronic officials have predicted they’ll be one of the first companies to the U.S. market with a pulsed-field ablation catheter.