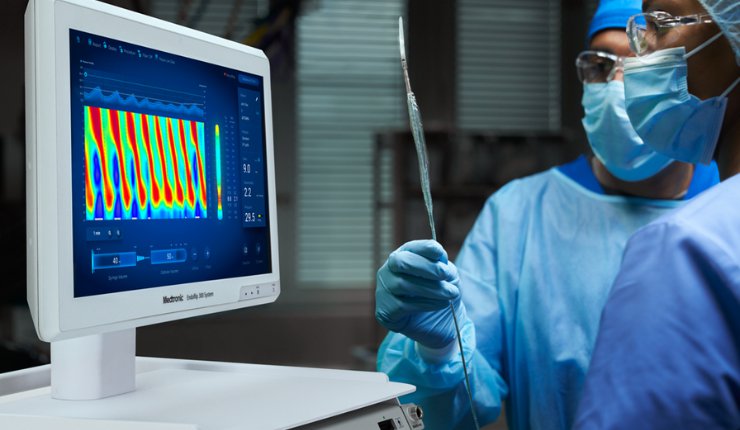
Medtronic has announced it has received CE Mark approval for its next generation Endoflip 300 system to measure pressure and dimensions in the oesophagus and pylorus in adults.
Medtronic says the Endoflip 300 System has the potential to help thousands of patients across Europe with oesophageal motility disorders achieve a diagnosis. The company says that research suggests these disorders are missed in up to 50% of endoscopies and patients can wait years to receive a diagnosis. Endoflip can be performed under sedation in around 5 minutes and can be utilised from the very first endoscopy.
Timely diagnosis of these conditions is key to improving patient outcomes. As pinpointing the underlying cause of patient symptoms can be very difficult, Endoflip represents a valuable tool as a convenient, well tolerated method for assessment.
The device uses a balloon catheter to display diameter estimates of the measurement area in real-time. It can measure and display diameter estimates at up to 16 points within the balloon while also displaying balloon pressure. This may allow for a more precise diagnosis and treatment planning from the very first endoscopy, preventing unnecessary distress for patients.