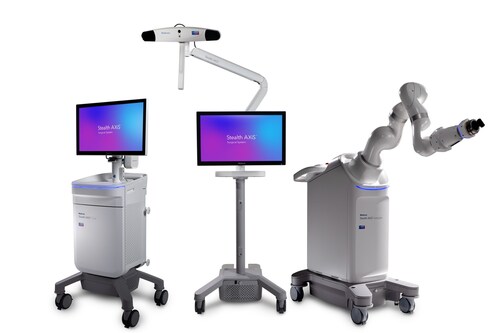
The Stealth AXiS™ system is cleared for spine procedures in the United States, with an underlying architecture designed to support future cranial and ENT applications, pending 510(k) clearance. Built to support a wide range of surgeon preferences, clinical complexity, and care settings, the platform is designed for use across hospitals and ambulatory surgery centers without relying on multiple standalone technologies.
Grounded in more than 50 years of Medtronic leadership in surgical navigation and robotics, the Stealth AXiS™ system creates a clear pathway for adoption by combining familiar navigation workflows with a modular robotic design, allowing institutions to deploy what they need today and expand over time as clinical needs evolve.