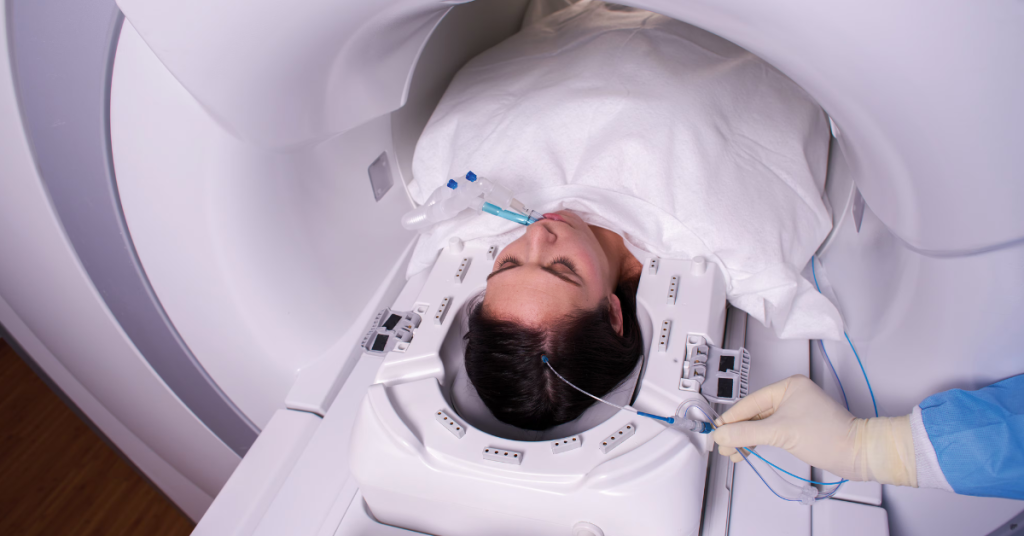
Visualase allows surgeons to navigate deep within the brain with pinpoint accuracy, gently delivering laser energy to ablate diseased tissue without disturbing surrounding structures. This is done through an incision just millimeters wide. Visualase uses laser interstitial thermal therapy (LITT) to deliver precisely targeted energy through a small catheter, enabling surgeons to ablate soft tissue under real-time MRI guidance. Compared to traditional open neurosurgery, the Visualase system offers several patient benefits:
- Minimally invasive access through a small (4 mm) incision
- Reduced hospital stays and faster recovery
- Minimal scarring
- Lower risk of infection
- High patient satisfaction
“This clearance is a significant advancement for patients and clinicians alike,” said Dr. Ashwini Sharan, chief medical officer of Medtronic Neuromodulation, which is part of the Neuroscience Portfolio. “By providing a minimally invasive option with real-time MRI guidance, we’re enhancing surgical precision. This is an important advancement for neurosurgical procedures.”