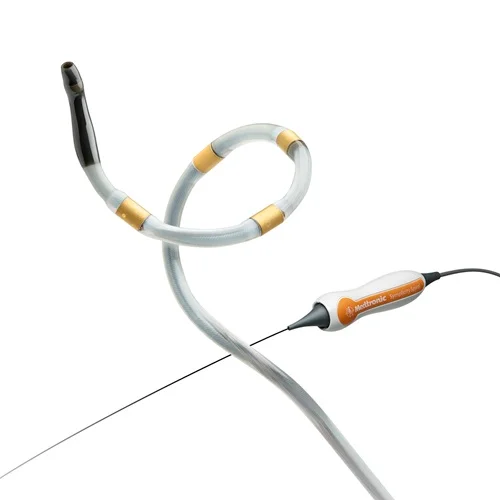
Medtronic’s announcement regarding the proposed National Coverage Determination (NCD) by the Centers for Medicare & Medicaid Services (CMS) marks a pivotal advancement for hypertension care in the United States. The inclusion of the Symplicity Spyral™ renal denervation (RDN) system in this proposal signifies federal recognition of its clinical relevance in addressing uncontrolled hypertension-a condition that affects nearly half of U.S. adults and is often resistant to traditional therapies. The CMS proposal not only validates the innovation behind Medtronic’s RDN platform but also paves the way for broader patient access through Medicare, especially for populations most burdened by this chronic disease.
The Symplicity Spyral RDN system is a minimally invasive, catheter-based procedure that uses radiofrequency energy to disrupt overactive renal nerves, which play a role in blood pressure regulation. This novel approach provides an adjunctive option for patients who do not adequately respond to lifestyle changes and medication. With the proposed NCD in place, clinicians would be empowered to offer RDN to a wider patient base, potentially improving long-term cardiovascular outcomes while reducing the economic and clinical strain of unmanaged hypertension.