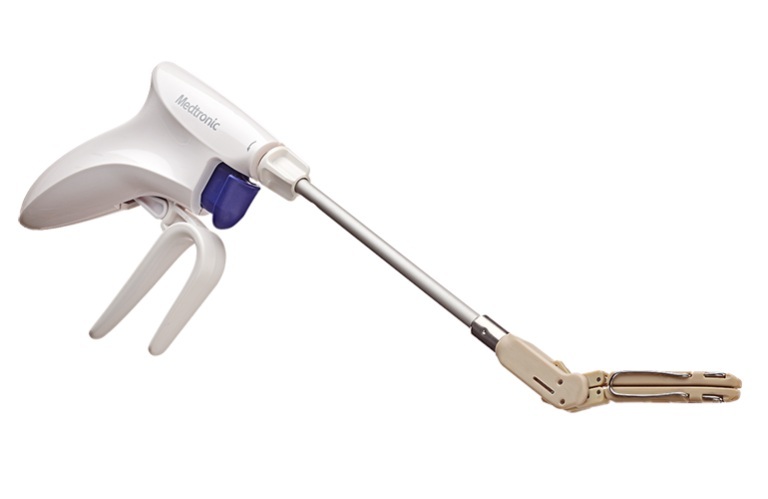
The company plans to begin the European launch of the left atrial appendage (LAA) exclusion device at the 39th European Association for Cardio-Thoracic Surgery (EACTS) Annual Meeting from October 8-11 in Copenhagen, Denmark. This regulatory milestone comes shortly after the device surpassed 10,000 clips sold globally.
Penditure, an implantable clip, comes pre-loaded on a single-use delivery system for use in LAA management during concomitant cardiac surgery procedures. Medtronic — one of the largest medtech companies in the world — designed Penditure with a curve to better match the atrial anatomy. It also has no fabric to reduce the risk of inflammation.