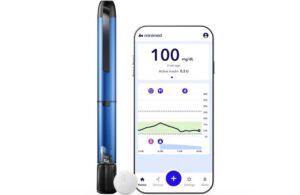
MiniMed Go, a smart MDI system, integrates the InPen smart insulin pen with the Instinct sensor made by Abbott. It all integrates within the app to deliver real-time, personalized insights and actionable guidance.
Medtronic and Abbott entered a collaboration in August 2024 to pair insulin delivery and continuous glucose monitoring (CGM) technologies. Medtronic (soon to be a standalone company called MiniMed) launched the MiniMed 780G automated insulin delivery system with the Instinct sensor made by Abbott late last year.