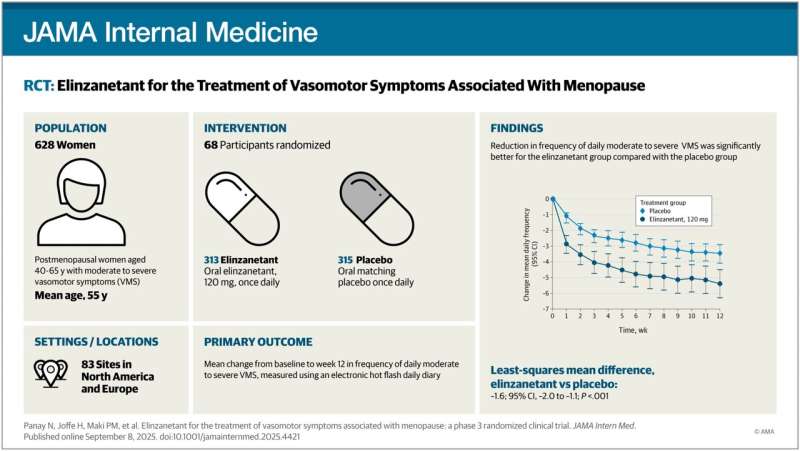
The OASIS-3 trial enrolled more than 600 postmenopausal women, ages 40 to 65, at 83 sites in North America and Europe. Participants were given either 120 mg of elinzanetant or a harmless placebo daily for 52 weeks.
Elinzanetant recipients saw a more than 73% reduction in the frequency and severity of “vasomotor symptoms”—hot flashes and night sweats—by week 12, the researchers report. The trial also found secondary benefits such as a reduction in sleep disturbances and overall improvements in reported quality of life, though the study did not have sufficient scope to assess these secondary benefits fully.
The drug had no harmful effects on the liver or bone density, the researchers determined.