The approval makes Alwide Plus the company’s fourth product authorized in Europe, following the VitaFlow Liberty transcatheter aortic valve and delivery system, and the AnchorMan left atrial appendage closure system with its access system.
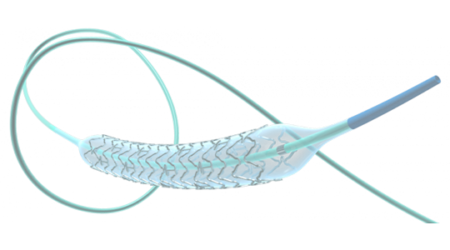
Alwide Plus is used in transcatheter aortic valve implantation (TAVI) procedures. The balloon catheter has been introduced in more than 10 countries, including China, Brazil, Thailand, Russia, and Mexico.
MicroPort CardioFlow designed Alwide Plus with ultra-low compliance for precise balloon dilation and high burst pressure for use in severely calcified anatomies. The catheter also allows for rapid inflation and deflation to shorten pacing time and reduce procedural risk.