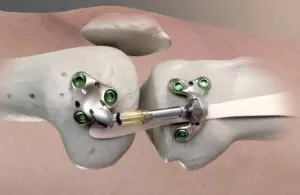
In April, the FDA granted Misha de novo clearance for the treatment of medial knee osteoarthritis (OA). Its indication covers people with medial knee OA who failed to find relief from non-surgical or surgical treatment. These patients continue to experience pain that interferes with daily activities. Patients eligible for Misha are ineligible for — or unwilling to undergo — joint replacement due to age or absence of advanced OA.
Fremont, California-based Moximed says Misha represents the world’s first implantable shock absorber (ISA) for the knee.
“We’ve had unsolicited online requests for access to the Misha knee system from over one hundred surgeons across the U.S., and we receive several online patient inquiries every day,” said Anton Clifford, founder and CEO of Moximed. “Despite this broad enthusiasm, our rollout plan remains focused on delivering high-quality education to our surgeon users, and I’m delighted to see our first surgeons represent both top-tier academic centers and high-caliber private practices.”