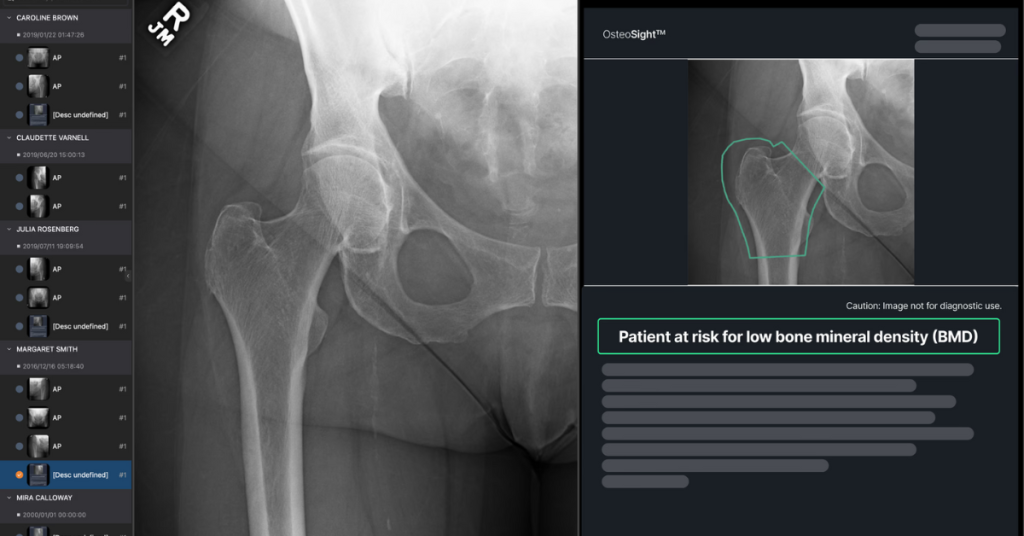
Osteoporosis, characterised by the progressive loss of BMD, impacts millions of Americans and costs healthcare systems billions each year. Yet, 70% of patients with low BMD are undiagnosed often until a fracture occurs. Earlier diagnosis drives earlier intervention, improving outcomes and reducing the condition’s impact. OsteoSight is designed to enable orthopedic practices to take a proactive role in osteoporosis management, supporting earlier interventions, and new growth opportunities for practices.
The device earned designation as an FDA Breakthrough Device in 2023 and has been validated in peer-reviewed research. Developed with a range of world-leading partners and front-line clinicians, OsteoSight addresses an urgent unmet clinical need, with a solution that fits seamlessly into the existing orthopedic workflow.