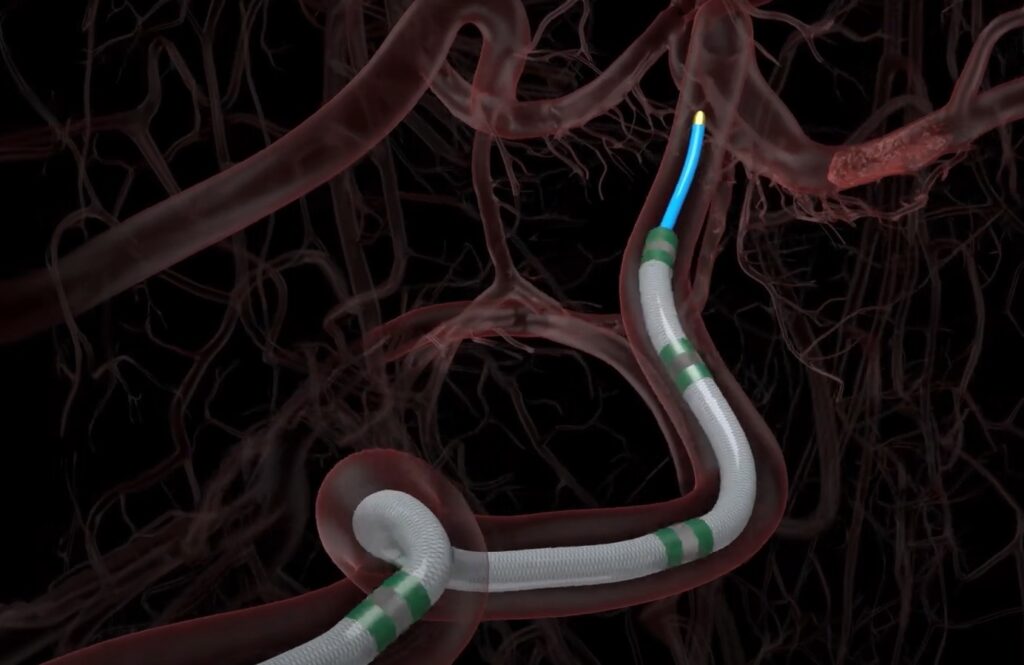
ZURICH, Switzerland, Jan. 10, 2024 (GLOBE NEWSWIRE) — Nanoflex Robotics AG, a remote robotic surgical company based in Switzerland, has received the internationally recognized ISO 13485 certification for its quality management system in designing and manufacturing remote robotic devices for endovascular interventions.
The ISO 13485 certification is the global standard for quality management systems within the medical device industry. To become certified, companies must demonstrate that important components are designed and manufactured in accordance with stringent regulatory standards.
The certification represents Nanoflex Robotics’ commitment to upholding high standards throughout its entire operational chain, from the initial phases of research and development to design and manufacturing. It is a step forward in bringing to market innovative remote robotic solutions that are designed to increase access to critical care procedures, such as the treatment of acute ischemic stroke.