The Copenhagen, Denmark–based company said the milestone “marks one of the most significant advances in resuscitation since the introduction of CPR in 1960.”
According to a news release, every year, an estimated 81% of cardiac arrest patients present as non-shockable. This makes them ineligible for defibrillation. Today, only 4% of these patients survive, the company said.
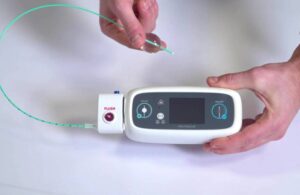
Neurescue designed its device to directly address this urgent unmet need. The company said CE mark approval makes it the first device of its kind made available anywhere in the world for non-shockable cardiac arrest treatment.
Consisting of a catheter and a handheld control unit, the device temporarily inflates a soft balloon in the descending aorta. This redirects blood flow toward the heart and brain, supercharging circulation to the most critical organs during CPR. The intelligent, fluoroscopy-free device can boost central blood flow within one minute of deployment.