TAMPA, Fla., Jan. 9, 2024 /PRNewswire/ — Neurolief, a global leader in medical devices delivering groundbreaking neurotechnology innovations for the treatment of Neurological and Neuropsychiatric disorders, announces the approval of its neuromodulation device, Relivion®, by the Japanese Ministry of Health, Labor and Welfare (MHLW). This approval marks a historic moment for Neurolief and its partner Sawai Pharmaceutical Co., Ltd, as Relivion® becomes the first neuromodulation device approved in Japan for at-home use in the acute treatment of migraine.
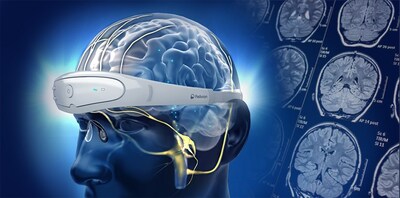
Relivion® Brain Neuromodulation Therapy
Relivion® is a novel, non-invasive multi-channel brain neuromodulation technology. It stands out by concurrently stimulating the occipital and trigeminal nerve branches in the head, effectively modulating brain networks associated with debilitating migraine headaches. The system is complemented by a state-of-the-art patient mobile app and a physician interface featuring cloud-enabled data-tracking capabilities, allowing seamless AI integration and enabling remote patient monitoring.