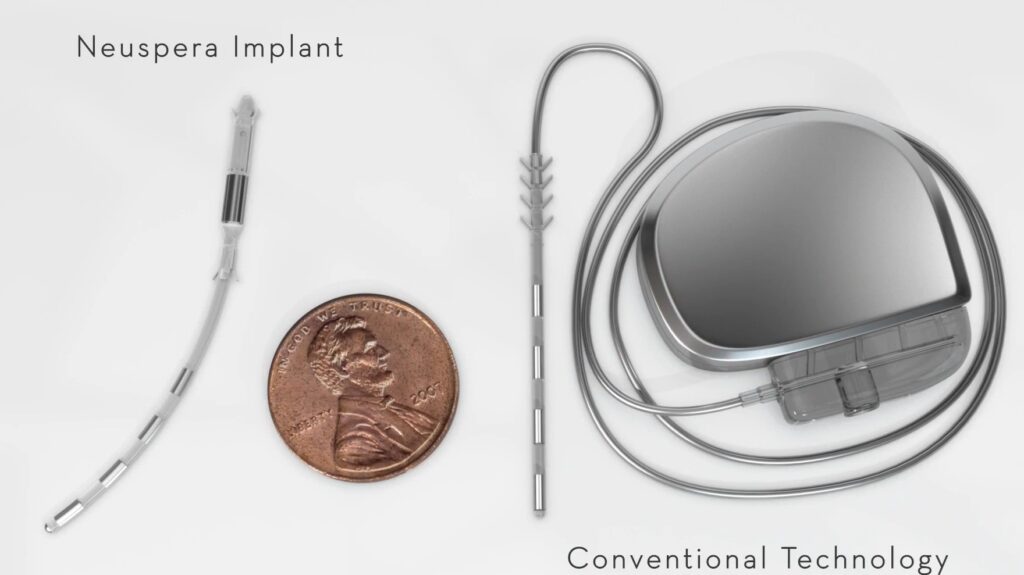
Neuspera’s iSNM system offers patients with UUI an alternative to traditional SNM. UUI is a major component of overactive bladder, a condition that affects approximately 1 in 5 women in the U.S. UUI has a major impact on quality of life, driving the demand for more effective and patient-friendly treatment options.
Six-month pivotal trial data showed the Neuspera iSNM device delivers efficacy comparable to established SNM therapies, while eliminating the discomfort, surgical risks, and cosmetic concerns of implanted batteries.1,2 The Phase II pivotal clinical study of 128 patients implanted with Neuspera’s iSNM therapy found:
- 84.2% of patients had a 50% or greater reduction in urgent leaks3 – on par with reported rates in recent SNM studies1,2
- 84% of patients who responded to treatment were classified as “super responders,” meaning they experienced more than a 75% reduction in UUI symptoms
- 42% of patients who responded were completely “dry,” with 100% reduction in UUI symptoms
- 3.5x clinically significant improvement in quality of life with a reduction in voids and urgent episodes