The Solana Beach, California-based company also reported the first-in-human cases following the FDA clearance.
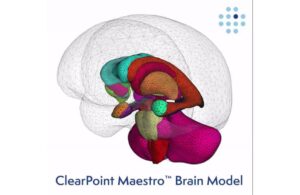
ClearPoint Neuro President and CEO Joe Burnett said the system now offers fast, peri-procedural segmentation of the cortical structures of the brain. This helps to identify targets and safety zones for cell and gene therapy delivery, laser ablation, biopsy and deep brain stimulation.
According to the company, MRI-guided neuro interventions need rapid, accurate and reproducible segmentation of brain structures. Maestro demonstrates superior performance compared to both manual expert segmentation and FreeSurfer, according to quantitative analysis.
The company expects a full market release of ClearPoint 2.2 in the second half of 2024.