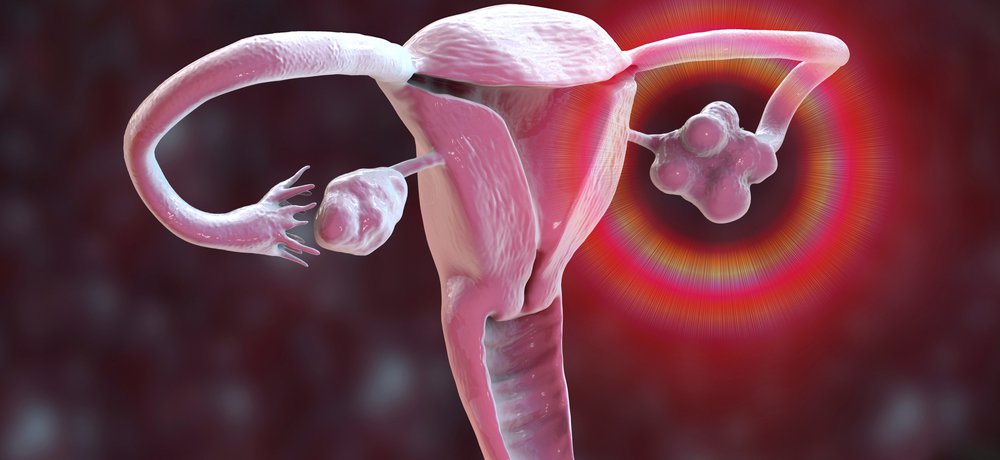
The test, which is currently used as a fertility indicator, identifies the presence of polycystic ovarian morphology (PCOM) in women with suspected PCOS. PCOM is an indicator of PCOS.
The claim extension follows an update in 2023 to the Rotterdam Criteria, the global guidance for diagnosing PCOS. The guidance now recommends that elevated AMH levels can be used to detect PCOM. Previously the only recommended indicator for PCOM was counting the number of follicles per ovary by transvaginal ultrasound.
PCOS affects an estimated one in eight women of reproductive age, and has reproductive, metabolic, and psychological consequences. Up to 70% of women living with PCOS remain undiagnosed.
The blood test aims to enable more patients with suspected PCOS to receive an easier and faster diagnosis while removing barriers often posed by transvaginal ultrasounds such as discomfort, and cultural sensitivities.
Many women suffering from PCOS are not diagnosed or diagnosed at a late stage. While there is no cure for PCOS, earlier diagnosis of the disease can ensure patients receive targeted therapy and are encouraged to make lifestyle choices to reduce the risk of developing long-term conditions, such as type 2 diabetes.
Dr Ashton Harper, head of medical affairs at Roche Diagnostics UK and Ireland, said: “I’m really pleased that we’re able to offer this innovative use of the Roche Elecsys Anti-Müllerian Hormone (AMH) Plus immunoassay as a more accessible alternative for diagnosing PCOS.