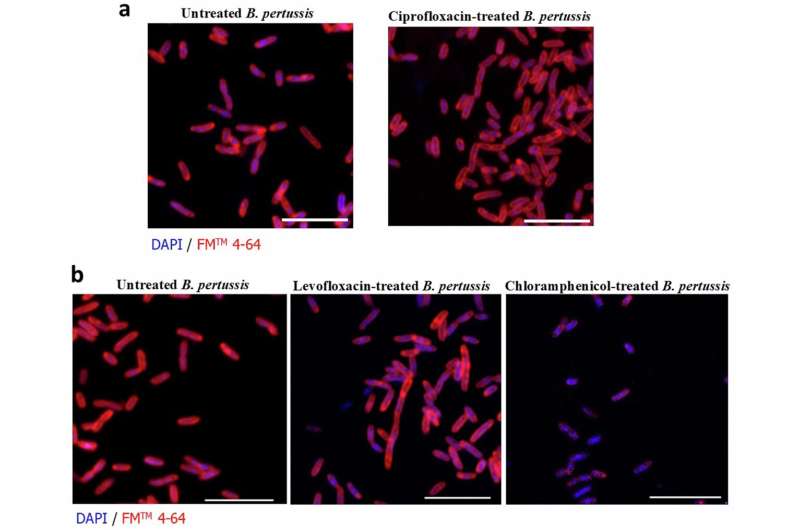
The work, led by Professor Kingston Mills and Dr. Davoud Jazayeri of Trinity’s School of Biochemistry and Immunology, introduces a needle-free mucosal vaccine platform capable of inducing durable local immunity directly at the infection site. This strategy could transform both whooping cough prevention and the broader market for respiratory bacterial vaccines, addressing an urgent global need for next-generation immunization technologies.
“We’ve applied our understanding of protective immune pathways to engineer a fundamentally different kind of vaccine,” said Prof. Mills.
“By stimulating immunity where infections begin, at the respiratory mucosa, we can offer stronger protection and potentially interrupt community transmission.”
Current whooping cough vaccines, while life-saving, have key limitations: they protect infants from severe illness but fail to prevent bacterial colonization in the nose and throat, allowing continued spread within communities. Global resurgence of pertussis—despite high vaccination coverage—underscores the commercial and clinical demand for improved vaccines.