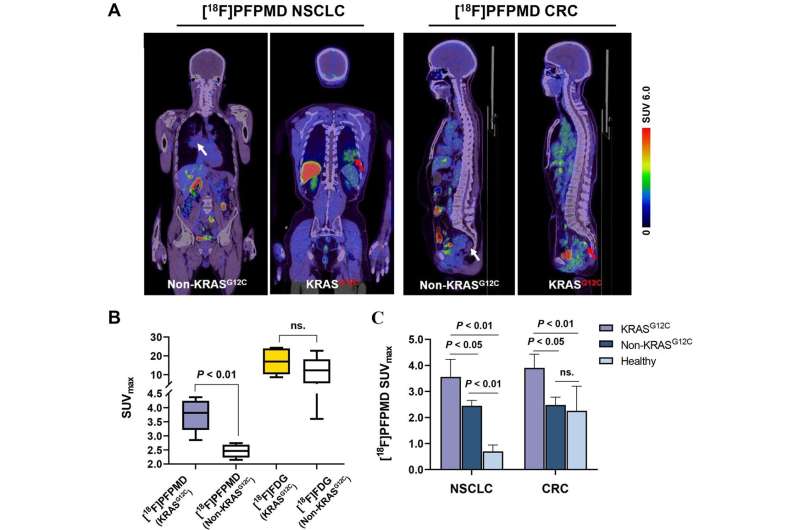
Kirsten rat sarcoma (KRAS) is a commonly mutated oncogene that is present in approximately 20%–70% of cancer cases. Patients with KRAS mutations usually respond poorly to standard therapies. As such, the National Comprehensive Cancer Network and other leading cancer research centers recommend assessing the mutation status in cancer patients to determine the most effective treatment.
“Currently, KRAS mutation screening relies on a biopsy combined with gene sequencing. However, biopsies have the potential for significant complications and their use is limited by the quality of the tissue sample. Thus, there is an urgent need for accurate yet noninvasive methods of evaluating the KRAS mutation status,” stated Jing Wang, MD, Ph.D., nuclear medicine physician at Xijing Hospital of Fourth Military Medical University in Xi’an, China.