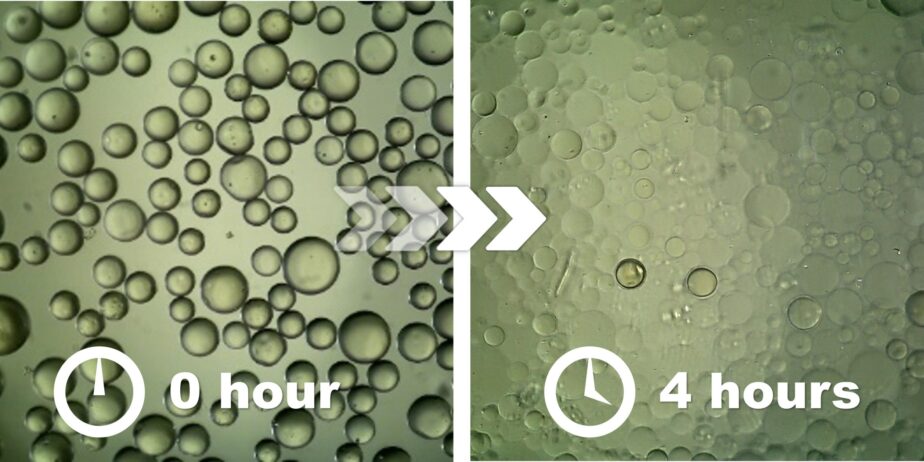
Nexsphere-F™ is a fast resorbable microsphere designed for musculoskeletal pain embolization. It temporarily occludes abnormal blood vessels associated with osteoarthritis-related pain using resorbable microspheres, inducing necrosis of pain-generating nerve cells within 2 to 8 hours after the procedure and effectively reducing pain. Unlike permanent microspheres, Nexsphere-F™ is designed to naturally dissolve after embolization, significantly lowering the risk of post-procedural complications and supporting the growing clinical demand for osteoarthritis pain embolization.
In addition to Canadian approval, Nexsphere-F™ is currently the only resorbable embolic microsphere certified under CE-MDD for osteoarthritis pain embolization, and is commercialized in major European countries. At CIRSE 2025, the annual congress of the Cardiovascular and Interventional Radiological Society of Europe, Dr. Florian Nima Fleckenstein (Charité-Universitätsmedizin Berlin, Berlin, Germany) presented, “Using rapidly resorbable, gelatin-based microspheres(Nexsphere-F™) for knee osteoarthritis pain embolization resulted in significant improvements in pain and function, while demonstrating favorable safety compared to permanent microspheres or antibiotic-based compounds.” These findings were recently reported by Interventional News, a leading interventional radiology news.