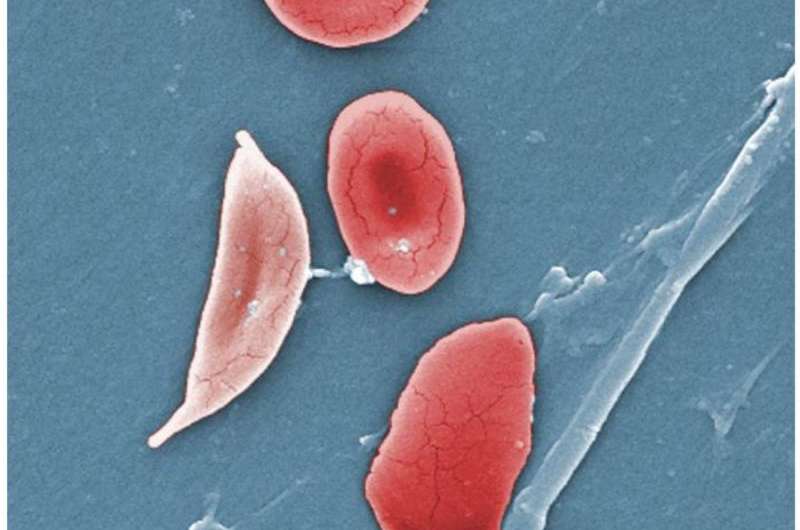
During this type of transplant, called reduced-intensity haploidentical bone marrow transplantation, bone marrow is given by a “half-matched” donor, such as a parent, sibling, child, niece, nephew, aunt, uncle or cousin of the patient. This means the proteins that help the body’s immune system function, and which are present on a donor’s marrow cells, must match at least half of those proteins on the recipient’s cells to be a good fit and to not attack the recipient’s body after the transplant.
Before the transplant, patients are treated with low doses of chemotherapy and given total body irradiation. Following the transplant, they are given cyclophosphamide (a drug to prevent graft-versus-host disease, in which immune cells in the donor marrow attack their new host) and other drugs for up to one year.