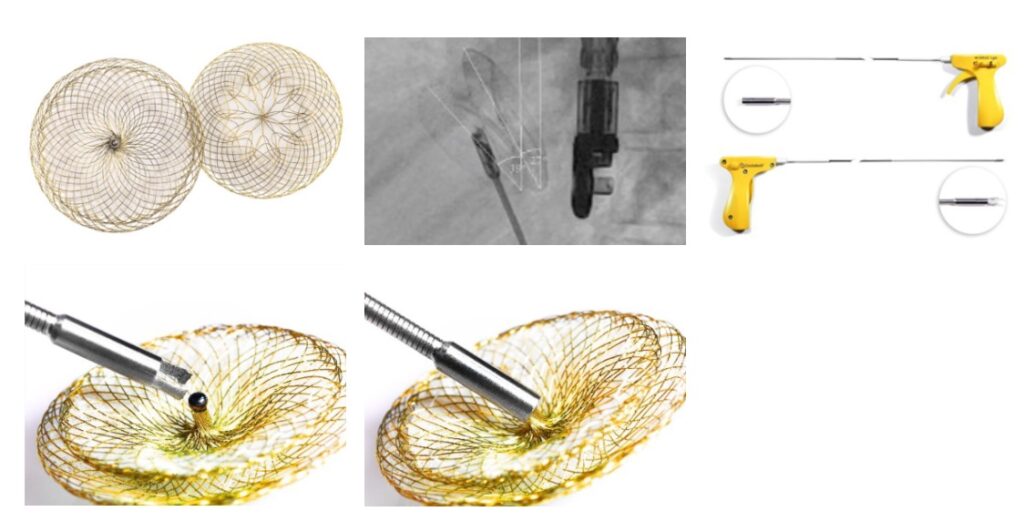
Occlutech®, a world leading specialist provider of minimally invasive structural heart implants, today announced that the United States Food and Drug Administration (FDA) has approved the Occlutech® ASD Occluder and Occlutech® Pistol Pusher for the treatment of Atrial Septal Defects (ASD). With this approval, Occlutech will immediately begin commercialization in an exclusive partnership with distributor B. Braun Interventional Systems Inc. (B. Braun Interventional Systems).
ASDs are one of the most common congenital heart defects (CHD) seen in pediatric cardiology[ii]. An ASD is a hole in the septum, or wall, between the two upper chambers of the heart (atria)[iii]. This opening causes abnormal blood flow between the atria and may result in too much blood flow to the lungs. If left untreated, an ASD can lead to fatigue, shortness of breath, pulmonary hypertension, heart failure, arrhythmia and/or an enlarged heart.
Sabine Bois, Occlutech CEO commented, “Today marks a momentous occasion for Occlutech and a significant leap forward in our commitment to advancing healthcare around the globe. I am thrilled the FDA has granted approval for the Occlutech ASD Occluder and Occlutech Pistol Pusher. Our mission has always been to improve the quality of life for patients; indeed, we have sold over 90,000 of our ASD devices outside of the U.S. Now, with the FDA’s approval, we are poised to leverage our experience in the largest congenital and structural heart disease market in the world, with ASD closure representing a $40 million market in the US with solid growth predicted.
Bois continued, “We extend our deepest gratitude to the patients, partners and all stakeholders who have been instrumental in this journey.”