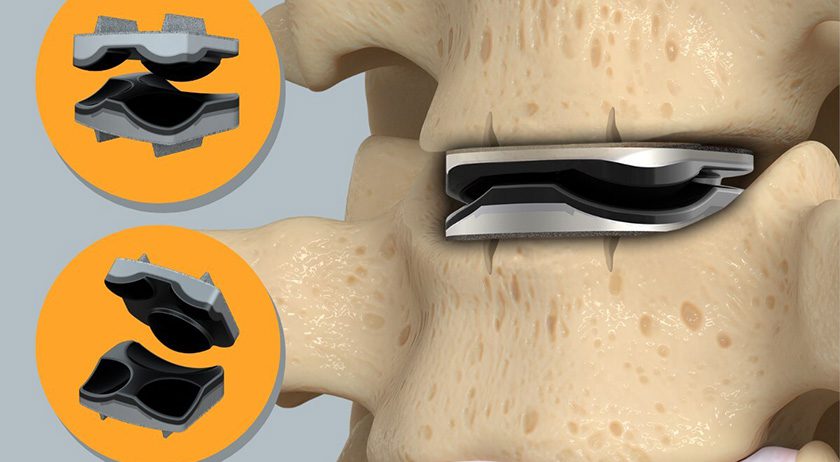
In May 2025, Omnia Medical received U.S. Food and Drug Administration (FDA) 510(k) clearance for the PsiF DNA™ System. Following clearance, the company conducted an initial alpha launch to gather physician feedback and refine commercial readiness. The success of this early launch provided Omnia with a springboard to proceed with a broader market introduction.
The PsiF DNA™ System is intended for sacroiliac joint fusion for treating conditions including degenerative sacroiliitis and sacroiliac joint disruptions. Thoughtfully engineered for the minimally invasive posterior approach, the system is designed to stabilize and promote fusion of the sacroiliac joint.
“The FDA clearance and commercial launch of PsiF DNA™ represent important milestones for Omnia Medical,” said Abigail Mann, Project Manager, SI Technologies. “They reflect our commitment to delivering physician-driven technologies that support procedural consistency while addressing the complexity of SI joint pathology.”