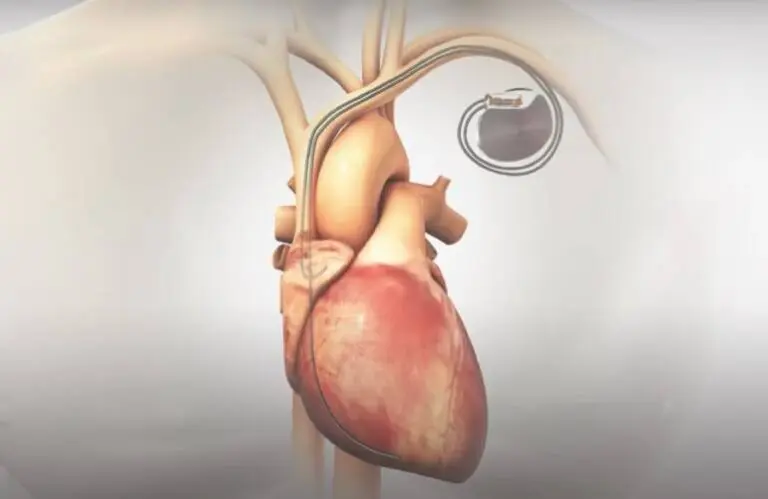
The breakthrough nod covers Orchestra BioMed’s implantable pacemaker system that delivers AVIM therapy. It uses conduction system pacing to reduce blood pressure in patients with increased 10-year atherosclerotic cardiovascular disease (ASCVD) risk, preserved left ventricular systolic function, and uncontrolled hypertension, despite the use of anti-hypertensive medications or in patients who may have intolerance to anti-hypertensive medications.
A patented bioelectronic therapy, AVIM, is administered using a standard dual-chamber pacemaker. Orchestra BioMed designed it to immediately, substantially and persistently reduce blood pressure.