It marks the second major regulatory milestone for Orchestra BioMed in just a week. The company last week picked up FDA breakthrough device designation for its atrioventricular interval modulation (AVIM) therapy.
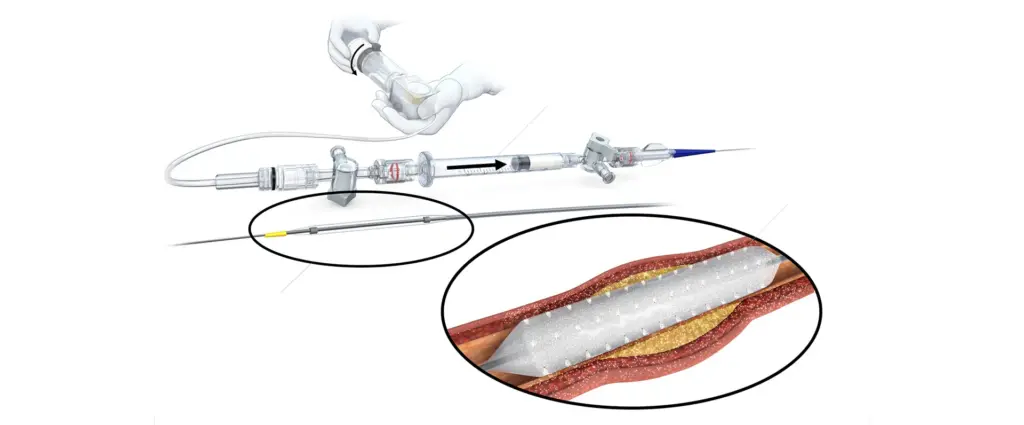
FDA IDE enables the company to initiate an updated design of its planned Virtue SAB in the Treatment of Coronary ISR Trial. The company plans to initiate a U.S. pivotal clinical trial comparing its next-generation Virtue SAB (sirolimus-angioinfusion balloon) to the Boston Scientific Agent paclitaxel-coated balloon. Agent is currently the only FDA-approved drug-coated balloon (DCB) for coronary indication, picking up approval last month.
Orchestra hopes to use the study results to support regulatory approval in the U.S.