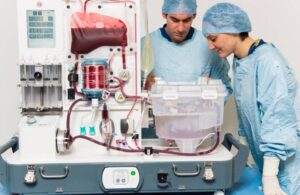
The company said approval supports the broader utilization of donor organs that might otherwise be discarded. Reasons for discarding could include time, geographic distance or marginal liver function. This milestone comes as Terumo works toward completing its planned acquisition of OrganOx in a deal announced last month.
NMP and other ex-vivo machine perfusion (EVMP) technologies can preserve organs longer by circulating oxygen and nutrient-rich perfusate through the organ at near-body temperature, Terumo says. They also enable real-time monitoring of organ condition during storage and transport. This can help prevent the transplantation of organs with impaired function and lead to more effective use of organs from marginal donors.