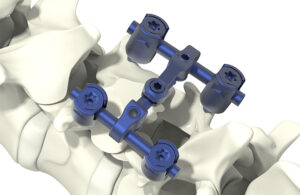
The Monmouth Junction, New Jersey-based device developer says it’s the first time the FDA has granted a de novo request for a non-eluting coating designed to actively kill bacteria that could contaminate the surface of a medical device.
Orthobond was founded by Princeton University Chemistry Professor Emeritus Jeffrey Schwartz and the late Dr. Gregory Lutz, who was physiatrist-in-chief emeritus at the Hospital for Special Surgery. Lutz died of cancer on March 5, but knew FDA approval was on the horizon.
Orthobond CEO David Nichols called the FDA approval a “testament to Gregory’s legacy” in a statement shared with MassDevice. “We are proud to be able to carry it on into this next chapter.”