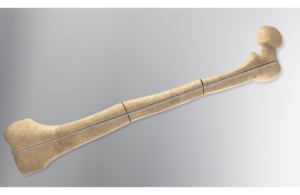
The Lewisville, Texas-based orthopedic company designed the Rodeo Telescopic Nail to surgically treat deformities or fractures in osteogenesis imperfecta (OI) patients. The nail implant stabilizes the patient’s limb while also elongating (or telescoping) to accommodate the natural growth of pediatric patients.
“The launch of the Rodeo Telescopic Nail represents Orthofix’s continued commitment to address the underserved pediatric market with specialized solutions tailored to the specific needs and unique conditions, such as OI, of this patient population,” Orthofix President of Global Orthopedics Kim Elting said in a news release. “The Rodeo system has been very well received in Europe, and we are pleased to be able to announce this limited U.S. market release during National OI Awareness Week and join in educating others about this genetic bone disorder that is present at birth.”