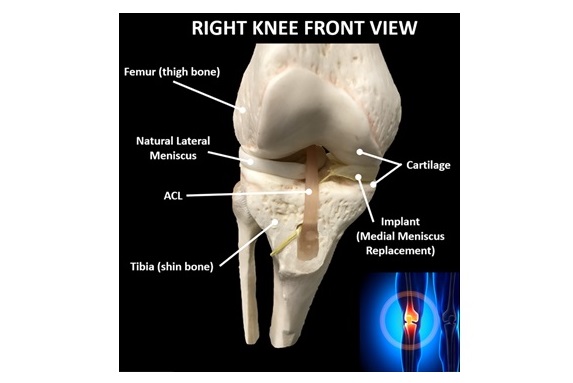
The designation covers the use of the therapeutic medical device to treat patients who continue to experience knee pain or impairment following a meniscus surgery, which impacts approximately 250,000 Americans annually. Prior to January 2025, orthopedic devices were not part of the TAP program, and OrthoPreserve’s device is believed to be the first Orthopedic device to win TAP enrollment.
OrthoPreserve’s implant addresses a significant treatment gap that exists due to the poor outcomes of the standard of care for meniscus tears, a partial meniscectomy, which removes the damaged tissue. OrthoPreserve’s proprietary technology has the potential to disrupt the standard of care and displace more invasive treatments.
“While current meniscus surgeries and non-operative care can relieve pain temporarily, some patients do poorly in the intermediate to long term, progress to further surgeries, and develop degenerative arthritis that eventually requires a knee replacement. OrthoPreserve’s implant has the potential to offer a minimally invasive, durable solution and higher quality of life for patients suffering from pain and mobility issues after a meniscus tear,” said Kenneth Zaslav, M.D., Director of the Center for Regenerative Orthopedic Medicine at Northwell Lenox Hill Hospital and Professor of Orthopedic Surgery.