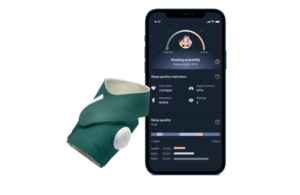
The over-the-counter Dream Sock delivers real-time health insights with medical-grade accuracy for at-home infant care. It received FDA clearance in November 2023. Owlet also raised $9 million to support its technology earlier this year.
With CE mark, the Lehi, Utah-based company plans to expand its medical device product portfolio around the world.
Dream Sock comfortably wraps around a baby’s foot and monitors and displays live health readings and provides health notifications. Readings include pulse rate and oxygen saturation level, while lights and alarms signal when readings fall outside of preset ranges. Its connected Owlet Dream App delivers live readings directly to a mobile device and nearby base station.