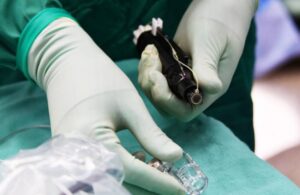
The FDA nod enables the company to start Connect-One, an early feasibility study (EFS) for the Connexus BCI system. Austin, Texas-based Paradromics says it marks the first IDE approval for speech restoration with a fully implantable BCI.
Connexus has the potential to help patients communicate after losing the ability to speak and control computer devices. It translates recorded brain signals into actionable health data. Initial focus centers around restoring independent communication through digital devices. It targets individuals living with spinal cord injuries, stroke, or ALS, a motor neuron disease.