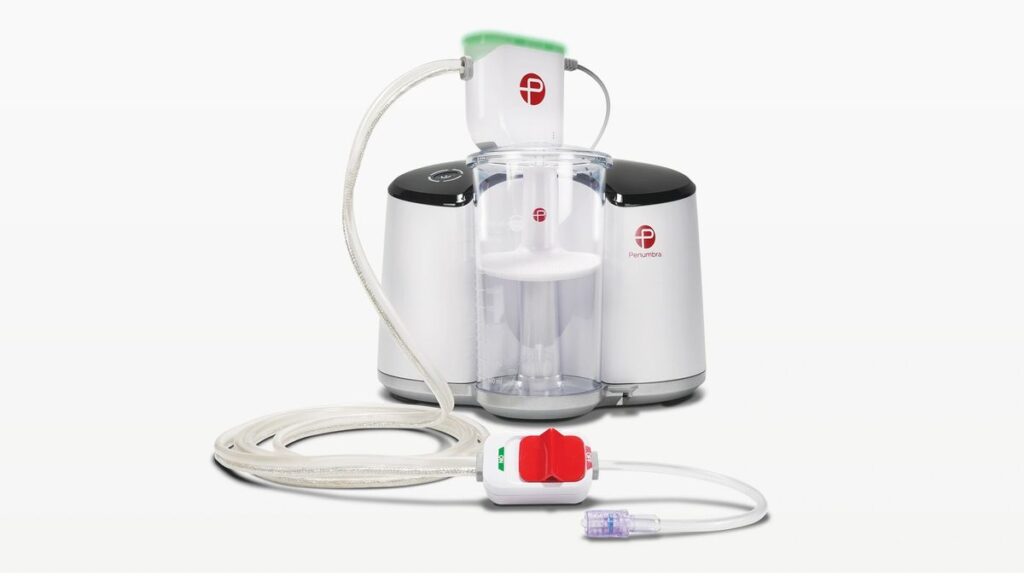
Penumbra shared interim results supporting the safety and effectiveness of its computer-aided vacuum thrombectomy system for treating pulmonary embolism.
The single-arm study showed a 25.7% reduction in the right ventricle/left ventricle ratio, a measure of right heart strain, using Penumbra’s Indigo aspiration system.
The company has another trial planned, called STORM PE, comparing its Indigo system paired with anticoagulants to taking anticoagulant drugs alone, BTIG analyst Ryan Zimmerman wrote in a research note.
Penumbra first received approval for its Indigo system with its Lightning technology in 2020. Earlier this year, the company received clearance from the Food and Drug Administration for its newer Lightning Flash technology to remove large clots while minimizing blood loss.
An interim analysis of 150 patients treated with Penumbra’s Indigo system with 90-day follow-up was presented at the Vascular Interventional Advances Conference (VIVA) on Wednesday.