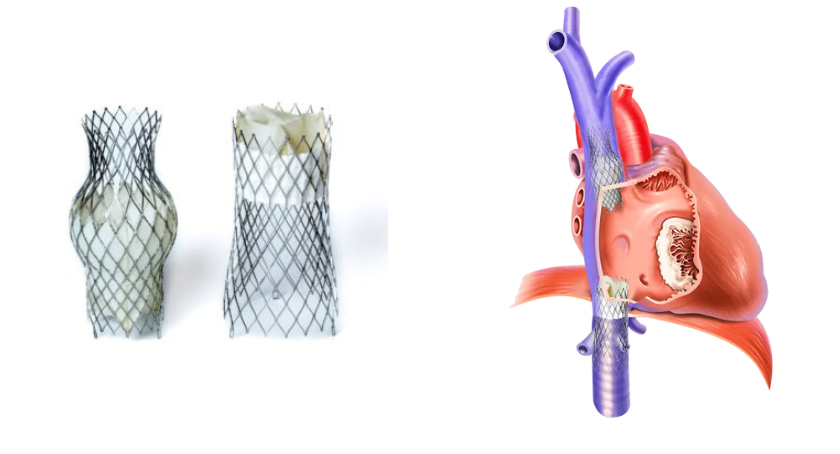
TRICAV II pivotal trial is a randomized, controlled study comparing the TricValve system with optimal medical therapy (OMT) versus OMT alone. The FDA previously authorized TRICAV I, the early feasibility study evaluating the TricValve system in 110 patients at 50 U.S. sites. TRICAV II advances its clinical development.
Severe TR affects an estimated 1.6 million people in the United States and is associated with progressive RHF, reduced quality of life, and frequent hospitalizations. Many patients are poor candidates for surgery due to frailty, comorbidities, or anatomical challenges. Others cannot be treated with currently available transcatheter therapies. As a result, a substantial patient population remains without a viable interventional option, relying solely on medical therapy with poor long-term outcomes.