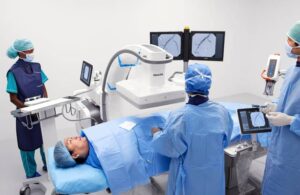
The Amsterdam-based medtech giant unveiled its image-guided therapy mobile C-arm system 9000, featuring Zenition 90. This new mobile C-arm features expanded capabilities aimed at meeting complex vascular needs. It also works with a range of clinical procedures, such as cardiac interventions, pain management and urology.
Philips plans to showcase its new mobile C-arm at the 2024 Society for Vascular Surgery Annual Meeting this week in Chicago. This latest launch follows the April FDA clearance of the company’s Zenition 30 mobile C-arm.