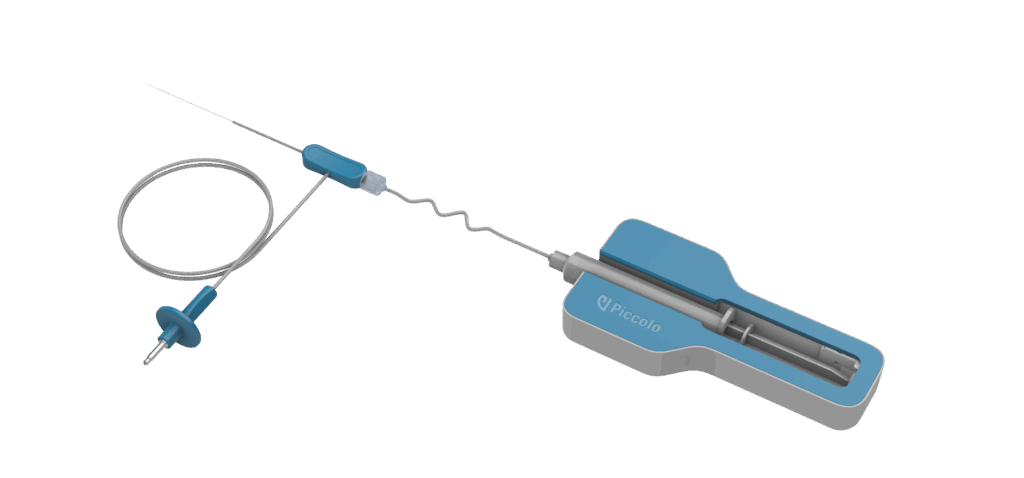
The SmartPICC® technology provides real-time, accurate bedside catheter tip confirmation for PICCs by leveraging the patient’s cardiac electrical activity. This well-established technique eliminates the need for confirmatory chest X-rays, streamlining the PICC confirmation procedure and reducing potential delays in patient care. The SmartPICC® Stylet also incorporates Piccolo’s proprietary ionic dilution technology for PICC navigation. This innovative feature provides clinicians with real-time guidance during catheter insertion, allowing for immediate detection of improper advancements through the vasculature.
“The landscape of PICC procedures in the U.S. has evolved, with catheter navigation systems becoming the undeniable standard of care,” stated Augustus Shanahan, CEO of Piccolo Medical, Inc. “However, the widespread adoption of these systems has often come with a significant financial burden on our healthcare system. We are incredibly proud to introduce a comprehensive vascular access solution to the market that not only prioritizes patient safety and positive outcomes but also empowers hospitals to realize the true economic value of bedside PICC confirmation.”