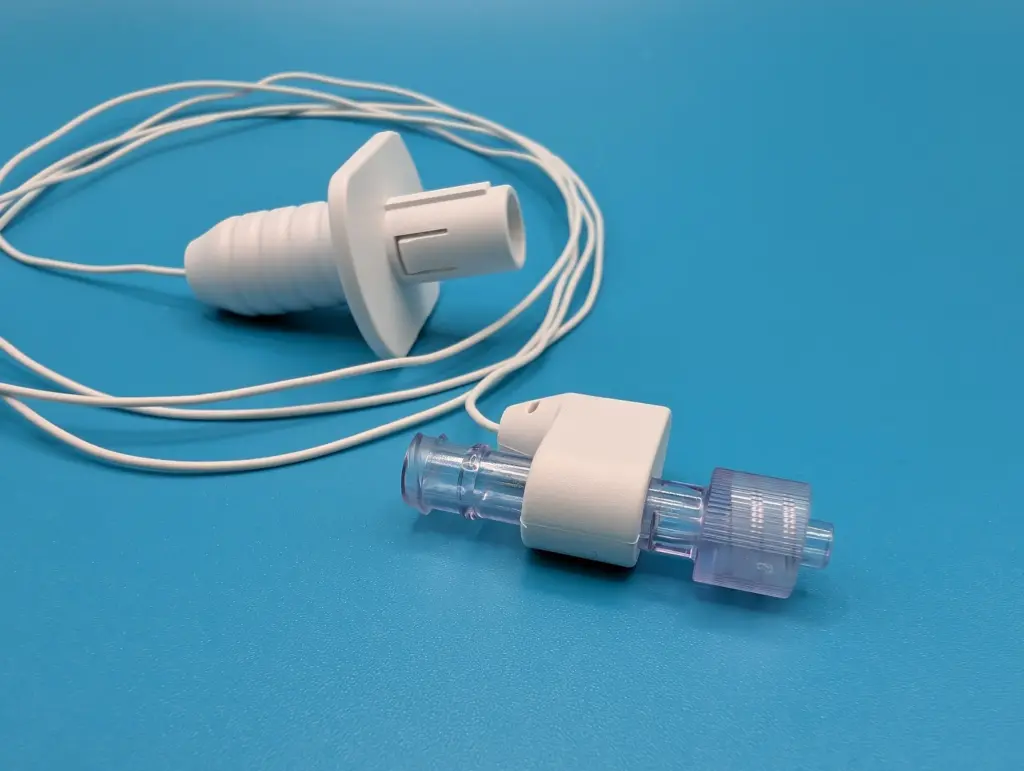
The ECGuide™ technology provides real-time, accurate catheter tip positioning by utilizing the patient’s cardiac electrical activity (ECG). This method provides an alternative to traditional radiological confirmation, significantly reducing the patient’s exposure to ionizing radiation and improving hospital efficiency. This benefit is especially critical for pediatric and neonatal patients, who are more vulnerable to the cumulative effects of radiation and often require multiple chest x-ray images for a single catheter confirmation.
The expanded indication allows for bedside confirmation of central venous access devices including peripherally inserted central catheters (PICCs), central venous catheters (CVC), hemodialysis catheters and ports. Bedside catheter tip confirmation using intravascular ECG has been shown to improve clinical workflow, avoid delays in therapy, and provide accurate tip position confirmation.