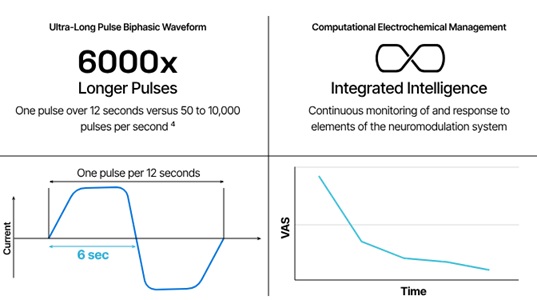
IDE enables the company to commence a global, pivotal, randomized controlled trial — the FULFILL study — in the U.S. and Australia. In the study, investigators intend to deliver ULF neuromodulation via leads implanted in the epidural space (spinal cord stimulation/SCS).
San Mateo, California-based Presidio designed its ULF neuromodulation platform to target localized pain by reducing neuronal responses. Suppression of these signals may reduce suffering for patients who have chronic nociceptive low back pain. The company says no current approved SCS treatment options exist for this type of back pain. It views it as a $20 billion market opportunity.