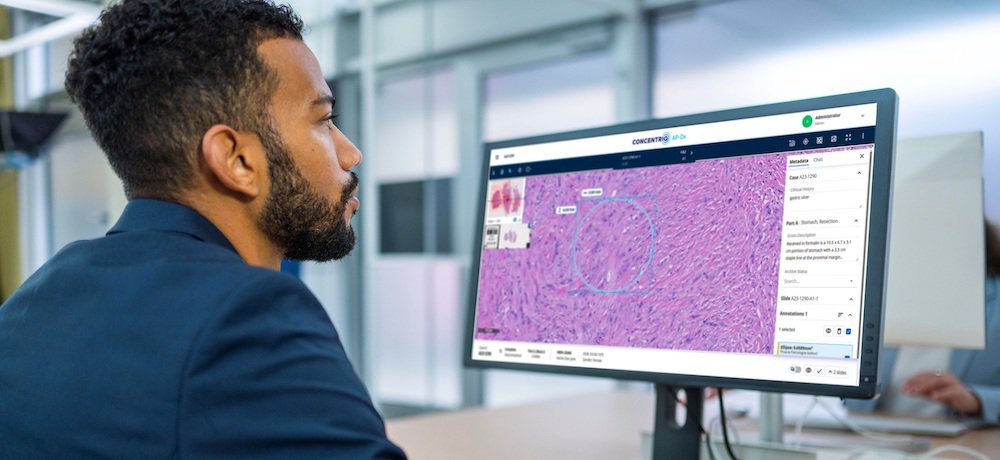
Proscia’s Concentriq AP-Dx is a diagnostic software solution that immerses pathologists in an intuitive experience for viewing, interpreting, and managing whole slide images and helps to drive confidence and efficiency gains. It can also streamline collaboration, broadening access to expertise. Concentriq AP-Dx was designed to be used in clinical settings of all sizes, from individual reference laboratories to the largest hospital systems.
In support of its 510(k) clearance, Proscia conducted a multi-site clinical study at PathGroup, South Bend Medical Foundation, and Spectrum Healthcare Partners. The study demonstrated that diagnoses made on Concentriq AP-Dx are non-inferior to traditional glass slide reads. Relative to ground truth data, the difference in major discordance rates for slides read digitally and for slides read using the microscope was -0.1%, which is among the strongest findings of similar publicly available studies.
David West, Proscia’s CEO, said: “This regulatory milestone reflects our tireless commitment to our mission of perfecting cancer diagnosis. Pathologists are facing more pressure than ever before in the fight against some of humanity’s biggest challenges. With 510(k) clearance, we can help more laboratories improve the pathologist experience and better serve their patients.”