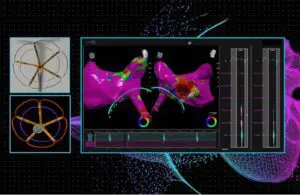
The Hayward, California-based company completed the first five procedures in its first-in-human feasibility study. Treating physicians successfully discharged all patients. They’ll continue to be monitored and evaluated over the coming months to assess safety and effectiveness. Pulse Biosciences plans to evaluate the primary safety endpoint at 30 days.
Dr. Vivek Reddy of Mount Sinai Hospital (New York) and Dr. Petr Neuzil of Na Homolce Hospital, Prague, and colleagues performed the procedures. They used the CellFX nxPFA (pulsed-field ablation) 360 catheter integrated with the CardioNXT iMap mapping and navigation. The doctors successfully treated five patients with AFib at the Czech Republic hospital.
“We have been collaborating with Pulse Biosciences to bring their novel nsPFA technology to the clinical realm, and are excited to report that our experience with these first five patients has validated our belief that this may represent the next generation of PFA technology for the treatment of AF,” said Dr. Reddy. “The results were consistent with our preclinical experience. Importantly, the speed and ease with which we were able to isolate the pulmonary veins with the nsPFA 360 catheter was impressive and all patients tolerated the procedure well. Now we look forward to completing enrollment in this study to fully assess the safety and durability of nsPFA treatment.”