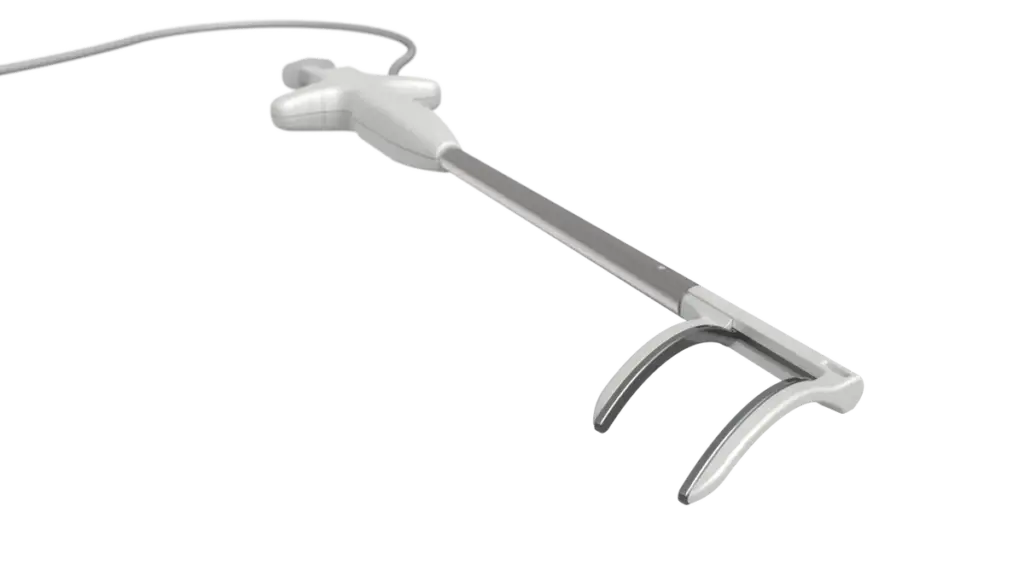
Pulse Biosciences said Monday it received Food and Drug Administration approval to begin a clinical study of its pulsed field ablation system to treat atrial fibrillation during concomitant cardiac surgeries.
The investigational device exemption clears the way for Pulse to start the NANOCLAMP AF study for its nsPFA cardiac surgical device. The single-arm prospective study, designed to demonstrate the system’s primary effectiveness, is expected to enroll as many as 136 patients at up to 20 sites, including two outside the U.S.
Pulse is the first company to advance PFA into the cardiac surgical field for the treatment of AFib, CEO Paul LaViolette said in a statement.