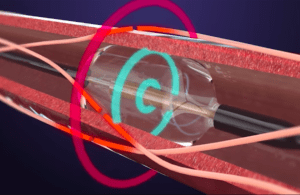
The long-awaited approval for the Otsuka Medical Devices portfolio company allows for the use of Paradise as an adjunctive treatment option when lifestyle changes and medications fail to adequately control a patient’s blood pressure.
Paradise had looked the most likely system to win approval in the race for the first FDA nod to use RDN to treat hypertension. An FDA panel voted in favor of FDA approving Paradise uRDN system in August. The same panel voted against recommending Recor’s closest competition, Medtronic’s Symplicity Spyral, a day later.
Recor presented a combined analysis of six-month follow-up data at last month’s Transcatheter Cardiovascular Therapeutics (TCT) symposium. The analysis demonstrated maintained blood pressure (BP) reduction following treatment with uRDN compared to sham. It also saw fewer antihypertensive treatments added in the treatment group. Paradise also met both primary safety and efficacy endpoints in the RADIANCE II trial, with results shared in February. RADIANCE II notably saw zero major adverse events.