SAN CLEMENTE, Calif.–(BUSINESS WIRE)– Reflow Medical, Inc., a developer of innovative medical devices focused on cardiovascular disease, announces it has received CE (Conformité Européenne) Mark certification in the European Union for the Bare Temporary Spur Stent System. The device is intended to treat de novo or restenotic lesions in the infrapopliteal arteries with a commercially available drug-coated balloon (DCB) to enhance drug absorption.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240116803175/en/
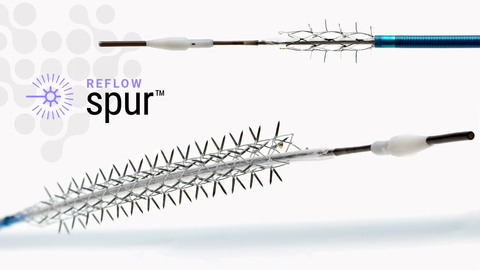
The Bare Temporary Spur Stent System is a unique clinical solution known as Retrievable Stent Therapy, or RST. It is intended to provide stent-like results while leaving no metal behind. (Graphic: Business Wire)
The Bare Temporary Spur Stent System is a unique clinical solution known as Retrievable Stent Therapy, or RST. It is intended to provide stent-like results while leaving no metal behind. (Graphic: Business Wire)
“The device performance and clinical study data for patients suffering from CLTI has been quite impressive,” said Professor Thomas Zeller, MD, who is Chief of the Department of Angiology at University Heart Center Freiburg in Bad Krozingen, Germany. CLTI, or chronic limb-threatening ischemia, increases the risk of mortality, amputation, and impaired quality of life. Prof. Zeller was a principal investigator in the DEEPER OUS clinical trial (NCT03807531).