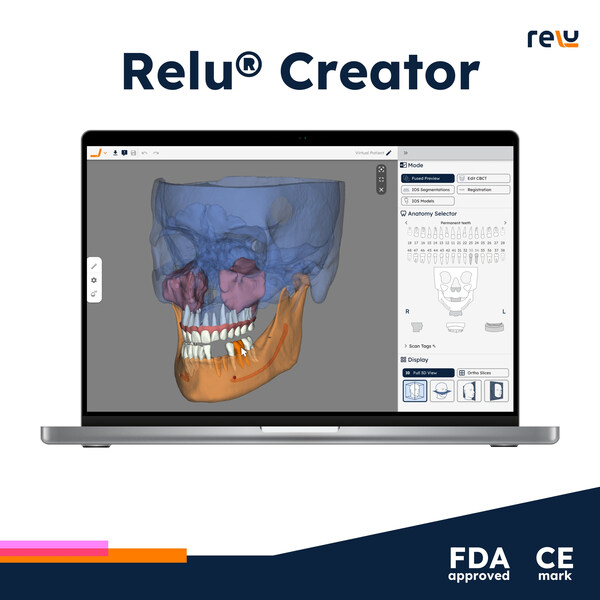
FDA clearance signifies compliance with U.S. medical device standards, while the CE Mark confirms adherence to EU regulations for product safety and consumer protection. This dual recognition underscores Relu’s commitment to excellence in the oral health sector.
“We are thrilled to have attained both FDA and CE approval for the Relu® Creator,” stated Adriaan Van Gerven, CTO and Co-founder, of Relu. “These significant achievements are a testament to Relu’s dedication to creating superior technology that meets the highest international safety and quality standards. We’re poised to revolutionize patient and dentist experiences across both continents.”