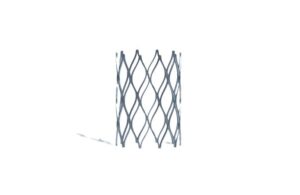
Newport Beach, California-based Renata designed the stent to re-expand over the course of the child’s growth period. The company says its approval marks a “groundbreaking advancement” in care for young children with congenital heart defects.
Renata won FDA approval for Minima in the treatment of vessel narrowing — or stenosis — in the aorta or pulmonary arteries. Narrowings can force the heart to work harder. When left untreated, a stenotic aorta or pulmonary artery can lead to heart failure and possibly death.