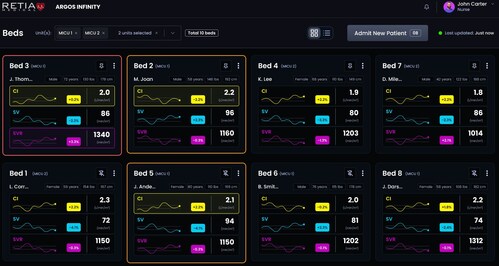
Argos Infinity builds on Retia’s clinically validated Multi-Beat Analysis® (MBA®) algorithm and analyzes real-time physiologic data streams from existing monitoring and tele-ICU systems. By transforming routine signals into actionable cardiovascular insight, Argos Infinity supports earlier recognition of hemodynamic instability in operating rooms and intensive care units.
High-risk patients often begin to deteriorate before changes appear in standard vital signs. In cardiac surgery patients, nearly 70% of low cardiac index time occurs while blood pressure remains normal. Delayed recognition can drive ICU escalation and organ injury, including acute kidney injury (AKI).