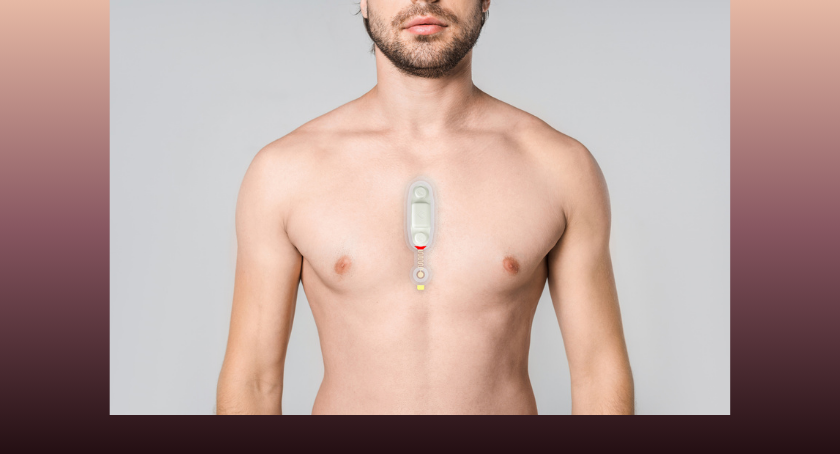
The company’s proprietary offering aims to transform cardiac rhythm management. It hopes to enable painless, imperceptible defibrillation and pacing through a minimally invasive approach.
Houston-based Rhythio designed its Injectable Electrode Gel as an alternative to traditional metal leads or shocking coils. The gel itself is the electrode — a soft, conductive hydrogel delivered through a needle directly to the target tissue. Once injected, the gel forms a stable electrical interface. It integrates seamlessly with existing implantable cardioverter defibrillators (ICDs) and pacemakers.
The Rhythio solution can convert conventional high-voltage shocks into gentle, patient-friendly therapies, the company says.