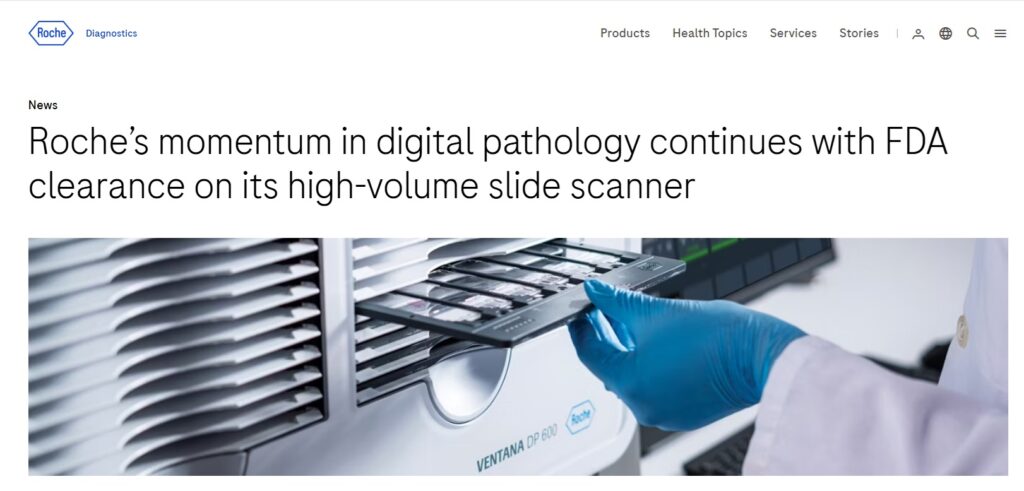
This clearance modifies the one Roche received on June 14, 2024 for Roche Digital Pathology Dx, which includes the VENTANA DP 200 slide scanner, Roche’s digital pathology workflow software and a display, and now adds the VENTANA DP 600 slide scanner.
“The VENTANA DP 600 high-capacity slide scanner creates high-resolution, digital images of stained tissue samples that help clinicians diagnose cancer and determine a patient’s treatment,” said Jill German, Head of Pathology Lab for Roche Diagnostics. “The recent FDA clearances continue our momentum to advance the pathology lab’s digital transformation and reinforce our commitment to enhance patient care and healthcare efficiency through streamlining the digital workflow.”
The VENTANA DP 600 has 40 times the capacity of the VENTANA DP 200 and uses the same scanning technology, providing pathologists with consistent, high quality images from both systems. Roche Digital Pathology Dx now includes the VENTANA DP 200 slide scanner, the VENTANA DP 600 slide scanner, Roche’s digital pathology workflow software and a display.